
HIV Test: HIV PCR RNA Test (HIV Polymerase Chain Reaction PCR) However, HIV PEP (Post-exposure prophylaxis) is effective in reducing the risk of HIV infection within 72 hours after potential HIV infection.ġ. There are No HIV Tests available within 10 days after exposure to HIV.
#Western blot test hiv accuracy registration
I confirm that any such study reported in the manuscript has been registered and the trial registration ID is provided (note: if posting a prospective study registered retrospectively, please provide a statement in the trial ID field explaining why the study was not registered in advance). I understand that all clinical trials and any other prospective interventional studies must be registered with an ICMJE-approved registry, such as. I confirm that all necessary patient/participant consent has been obtained and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone (e.g., hospital staff, patients or participants themselves) outside the research group so cannot be used to identify individuals. Swissethics gave ethical approval for this work (ref: 2018-01399). The International Agency for Research on Cancer gave ethical approval for this work(IEC project number 18-15). The Zambia Medicines Regulatory Authority gave ethical approval for this work (ref: DMS/7/9/22/CT/084). National Health Research Authority of University of Zambia Biomedical Research Ethics Committee gave ethical approval for this work (ref: 014-09-18). The details of the IRB/oversight body that provided approval or exemption for the research described are given below: I confirm all relevant ethical guidelines have been followed, and any necessary IRB and/or ethics committee approvals have been obtained. This work was supported by Swiss Cancer Research, grant number KFS-17, National Institute of Allergy and Infectious Diseases of the National Institutes of Health, grant number U01AI069924, and ESTHER Switzerland foundation, grant number 171222. The authors have declared no competing interest. Methodologically robust studies are crucial to inform cervical cancer screening practices and policies for the successful implementation of a cervical cancer elimination plan in sub-Saharan Africa, where 85% of women with cervical cancer and HIV live.
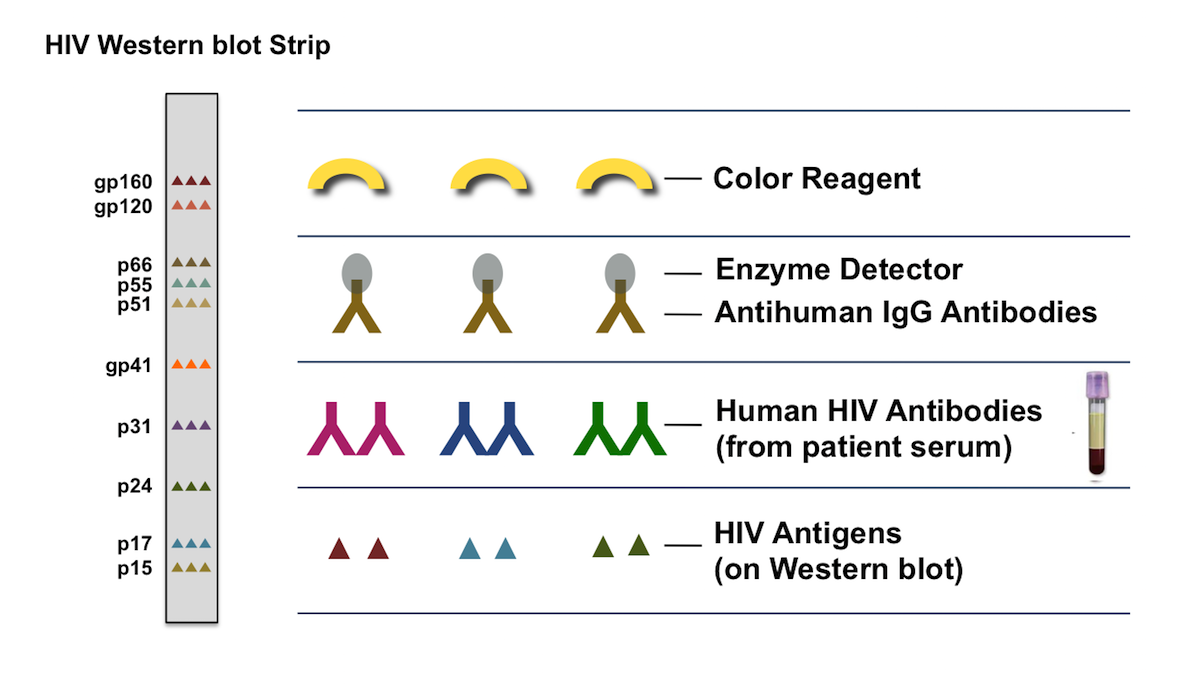
#Western blot test hiv accuracy verification
How this study might affect research, practice or policy Our findings have implications for research and cervical cancer screening policies among WLHIV if test-accuracy in this high-risk population has been overestimated from a majority of exsisting studies that are affected by verification and misclassification biases.


 0 kommentar(er)
0 kommentar(er)
